Explorando las propiedades físicas y químicas del litio
El litio es uno de los elementos más fascinantes de la tabla periódica. Conocido por su notable ligereza y alta reactividad, el litio desempeña un papel vital en la tecnología moderna, desde la propulsión de vehículos eléctricos hasta funciones críticas en aplicaciones médicas e industriales. En este artículo, profundizaremos en las propiedades físicas y químicas del litio, descubriendo por qué este elemento es tan indispensable. Comprender el litio no solo amplía nuestros conocimientos de química, sino que también nos permite apreciar su importancia crucial en la vida cotidiana.
- Introducción al elemento litio
- Propiedades físicas del litio
- Propiedades químicas del litio
- Aplicaciones derivadas de las propiedades del litio
- Características únicas que distinguen al litio
- Impacto ambiental y sostenibilidad del litio
- Perspectivas futuras de la investigación sobre el litio
- Medidas de seguridad en la manipulación del litio
Introducción al elemento litio
El litio, con número atómico 3, es el metal más ligero y el elemento sólido más ligero en condiciones estándar. Pertenece al grupo de los metales alcalinos y es altamente reactivo e inflamable. Fue descubierto en 1817 por Johan August Arfvedson y desde entonces ha adquirido una importancia creciente en diversas aplicaciones tecnológicas. Presente en minerales como la espodumena y la petalita, el litio debe extraerse mediante complejos procesos químicos.
El descubrimiento histórico del litio
La historia del litio es fascinante. Arfvedson identificó un nuevo componente al analizar el mineral petalita. Aunque no pudo aislar el elemento puro, su descubrimiento impulsó experimentos posteriores que finalmente condujeron al aislamiento del litio metálico. Con el paso de los años, el litio pasó de ser una curiosidad de laboratorio a un elemento comercialmente vital.
Fuentes comunes de litio
El litio se encuentra tanto en depósitos minerales como en salares. Las fuentes de litio más famosas se encuentran en el "Triángulo del Litio" de Sudamérica, una zona que abarca Argentina, Bolivia y Chile. Australia también es un productor líder, extrayendo litio principalmente de minas de roca dura.
>>Vea también ¿Cuántos vatios se necesitan para cargar una batería de 24 V?
Propiedades físicas del litio
Comprender las propiedades físicas del litio es crucial para comprender su comportamiento y sus aplicaciones. Aquí exploramos los aspectos esenciales de su naturaleza física.
Apariencia y textura
El litio tiene un aspecto blanco plateado recién cortado, pero se deslustra rápidamente al exponerse al aire. Es lo suficientemente blando como para cortarse con un cuchillo, una característica que comparte con otros metales alcalinos.
Densidad y peso
El litio es notablemente ligero, con una densidad de aproximadamente 0,534 gramos por centímetro cúbico, lo que lo convierte en aproximadamente la mitad de denso que el agua. Esta baja densidad lo hace ideal para aplicaciones que requieren materiales ligeros.
Puntos de fusión y ebullición
El litio tiene un punto de fusión de aproximadamente 180,5 °C (356,9 °F) y un punto de ebullición de unos 1342 °C (2448 °F). Estas temperaturas relativamente altas, en comparación con otros metales alcalinos, resaltan la singular estabilidad física del litio al calor.
Conductividad
El litio es un excelente conductor de calor y electricidad, una propiedad crucial para su uso en baterías y componentes electrónicos.
Propiedades químicas del litio
Las propiedades químicas del litio revelan su alta reactividad y versatilidad. Estas características lo convierten en un componente valioso en numerosos procesos industriales.
Reactividad con el agua
El litio reacciona vigorosamente con el agua para formar hidróxido de litio y gas hidrógeno. Sin embargo, es menos reactivo que otros metales alcalinos como el sodio o el potasio, lo que lo hace algo más seguro de manipular en entornos controlados.
Reacción con el aire
Al exponerse al aire, el litio forma una capa de óxido de litio que, en cierta medida, previene la corrosión. Sin embargo, debe almacenarse en aceite o en una atmósfera inerte para evitar una degradación significativa.
Compuestos formados por litio
El litio forma diversos compuestos, como el carbonato de litio, el cloruro de litio y el hidruro de litio y aluminio. Estos compuestos son esenciales en campos que van desde la medicina (como los fármacos estabilizadores del ánimo) hasta la industria aeroespacial (como propulsores de alta energía).
Aplicaciones derivadas de las propiedades del litio
Las propiedades físicas y químicas del litio han dado lugar a numerosas aplicaciones revolucionarias que impactan la vida cotidiana y las tecnologías emergentes.
Baterías de litio
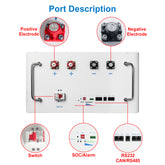
Las baterías de iones de litio y de polímero de litio dominan los mercados de la electrónica portátil y los vehículos eléctricos. Su alta densidad energética, ligereza y larga vida útil son resultado directo de las propiedades intrínsecas del litio.
El litio en la medicina
El carbonato de litio y otros compuestos de litio se utilizan para tratar el trastorno bipolar y otras afecciones mentales. El efecto del litio sobre el sistema nervioso ha sido un descubrimiento revolucionario en la medicina psiquiátrica.
Usos industriales y metalúrgicos
El litio se emplea para fortalecer aleaciones, mejorar lubricantes y crear vidrio y cerámica resistentes a altas temperaturas. Su combinación única de propiedades químicas y físicas lo hace insustituible en estas industrias.
Características únicas que distinguen al litio
La baja masa atómica del litio, su alto potencial electroquímico y su comportamiento químico distintivo lo distinguen de otros elementos. Estas características lo hacen especialmente valioso para el avance de las tecnologías de energía verde y la mejora del rendimiento de diversos productos.
Potencial electroquímico del litio
El alto potencial electroquímico del litio lo convierte en un candidato ideal para su uso en baterías recargables. Esta propiedad permite salidas de mayor voltaje y mayor capacidad de almacenamiento de energía.
El papel del litio en las aplicaciones nucleares
Debido a su bajo número atómico, el litio se utiliza en reacciones de fusión nuclear y como refrigerante en reactores nucleares. Su capacidad para absorber neutrones también contribuye a su importancia nuclear.
Impacto ambiental y sostenibilidad del litio
Si bien el litio ofrece numerosos beneficios, su extracción y uso plantean preocupaciones ambientales. Las prácticas sostenibles y las iniciativas de reciclaje son esenciales para minimizar la huella ecológica del litio.
Preocupaciones mineras y ecológicas
La extracción de litio, en particular de fuentes de salmuera, puede provocar el agotamiento del agua y la alteración de los ecosistemas. Las innovaciones en las técnicas mineras son cruciales para reducir el daño ambiental.
Reciclaje y reutilización del litio
El reciclaje de litio de baterías usadas y otros productos es una industria en expansión. Los esfuerzos para mejorar la eficiencia del reciclaje son cruciales para que el uso del litio sea más sostenible.
Perspectivas futuras de la investigación sobre el litio
La investigación sobre el litio continúa evolucionando, y los científicos exploran nuevas químicas de baterías, métodos de extracción alternativos y procesos de reciclaje mejorados.
Avances en baterías de litio
Las baterías de litio de estado sólido prometen soluciones de almacenamiento de energía más seguras y eficientes. Los investigadores también están estudiando las baterías de litio-azufre y litio-aire como tecnologías de próxima generación.
Nuevas tecnologías de extracción
Las técnicas innovadoras, como la extracción directa de litio (DLE), tienen como objetivo hacer que el abastecimiento de litio sea más respetuoso con el medio ambiente y rentable.
>>Vea también Cómo actualizar la batería de su carrito de golf con una guía completa de conversión de litio
Medidas de seguridad en la manipulación del litio
Dada su reactividad, la manipulación del litio requiere estrictos protocolos de seguridad.
Prácticas de almacenamiento
El litio debe almacenarse en entornos no reactivos, a menudo sumergido en aceite mineral o almacenado bajo una atmósfera inerte para evitar riesgos de oxidación e incendio.
Procedimientos de emergencia
En caso de incendio de litio, se requieren extintores especializados de clase D, ya que los extintores tradicionales a base de agua pueden exacerbar el incendio.
Conclusión Explorando las propiedades físicas y químicas del litio
La increíble variedad de propiedades físicas y químicas del litio refuerza su importancia en diversas industrias. Desde alimentar nuestros teléfonos inteligentes hasta habilitar vehículos eléctricos y estabilizar trastornos del estado de ánimo, el papel del litio en nuestras vidas es indispensable. A medida que la demanda de litio continúa creciendo, también crece la necesidad de prácticas sostenibles e investigación avanzada. Al comprender a fondo el litio, podemos apreciar mejor y aprovechar responsablemente este extraordinario elemento para el beneficio de las generaciones futuras. La trayectoria del litio, desde su descubrimiento hasta su dominio, es un testimonio de su importancia incomparable en el mundo moderno.






















Leave a comment
All blog comments are checked prior to publishing